GEICAM – delegated sponsor NL: BOOG
NL: M. (Maaike) de Boer, MUMC
S.M. (Susan) van den Berg, BOOG Study Center
Projectmedewerker: S. (Suzanne) Jager, BOOG Study Center
GEICAM
IKNL
ziekenhuis zelf
GEICAM
De studie is voortijdig gesloten. Overwegingen daarbij waren:
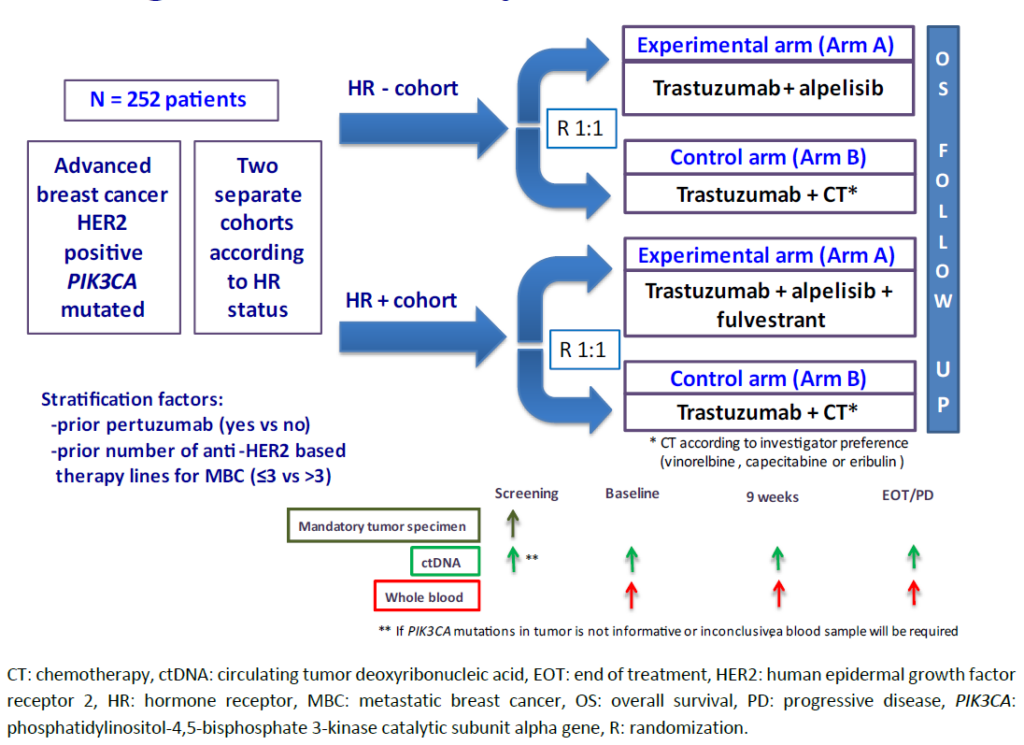
In Nederland zijn geen patiënten geïncludeerd en wereldwijd slechts 27 patiënten van de benodigde 252 patiënten. Hierdoor worden geen resultaten van deze studie verwacht.
International, multicenter, open-label, controlled phase III randomized clinical trial