
BOOG Study Center
Prof. dr. G.S. Sonke MD PhD (The Netherlands Cancer Institute)
Dr. A.E. van Leeuwen-Stok (BOOG Study Center)
F. Louis, MD E: TRAIN3@nki.nl
Registration: Authorized persons at the site, through ALEA
Central contact Data Center: K. Pengel
NKI-AVL Data Center Phone +31 20 512 2618 E-mail k.pengel@nki.nl
NKI-AVL Data Center
Phone +31 20 512 2655
E-mail e.v.schaffelaar@nki.nl
Centrum zelf of IKNL
E-mail: trialbureau@iknl.nl
Phone +31 88 234 6500
Roche
Contracts & finances: BOOG Study Center (info@boogstudycenter.nl)
Radiology: R. Mann, MD PhD: Ritse.Mann@radboudumc.nl
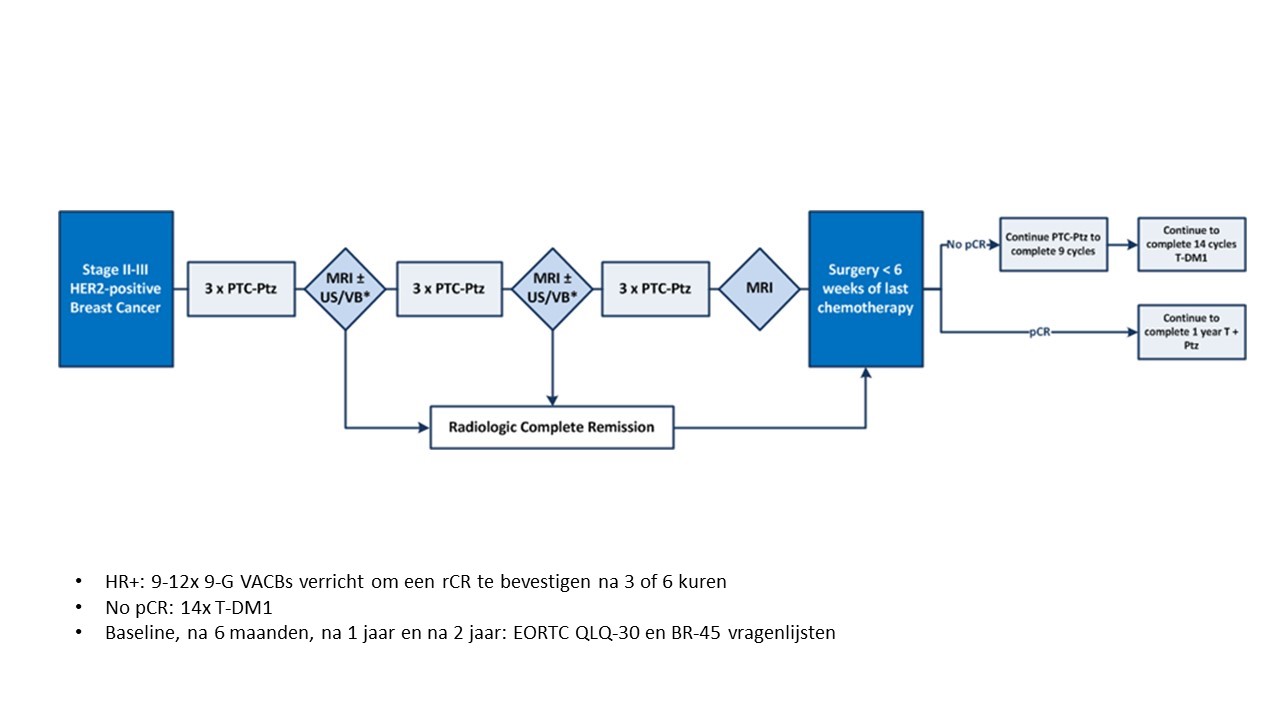
Primary efficacy objective
To evaluate efficacy of image-guided de-escalating chemotherapy in the presence of dual HER2-blockade with Herceptin® and pertuzumab in HER2-positive breast cancer, as measured by three-year event-free survival.
Secondary efficacy objectives
Patient-reported outcome objective
To compare health‐related quality of life after receiving 3, 6 or 9 cycles of chemotherapy using the EORTC QLQ‐30 and QLQ‐BR45 questionnaires
Safety objectives
Exploratory objectives
Primary efficacy outcome measure:
Event-free survival (EFS), defined as the interval from registration to the earliest occurrence of disease progression resulting in inoperability, invasive loco regional recurrence, distant metastases, or death from any cause, whichever comes first
Secondary efficacy outcome measures:
Patient‐reported‐outcome measure:
Health‐related quality of life scored using the EORTC QLQ‐30 and BR‐45 questionnaires
Safety outcome measures:
Exploratory outcome measures:
Eligible patients must meet all of the following criteria:
Exclusion criteria:
A subject who meets any of the following criteria will be excluded from participation in this study: